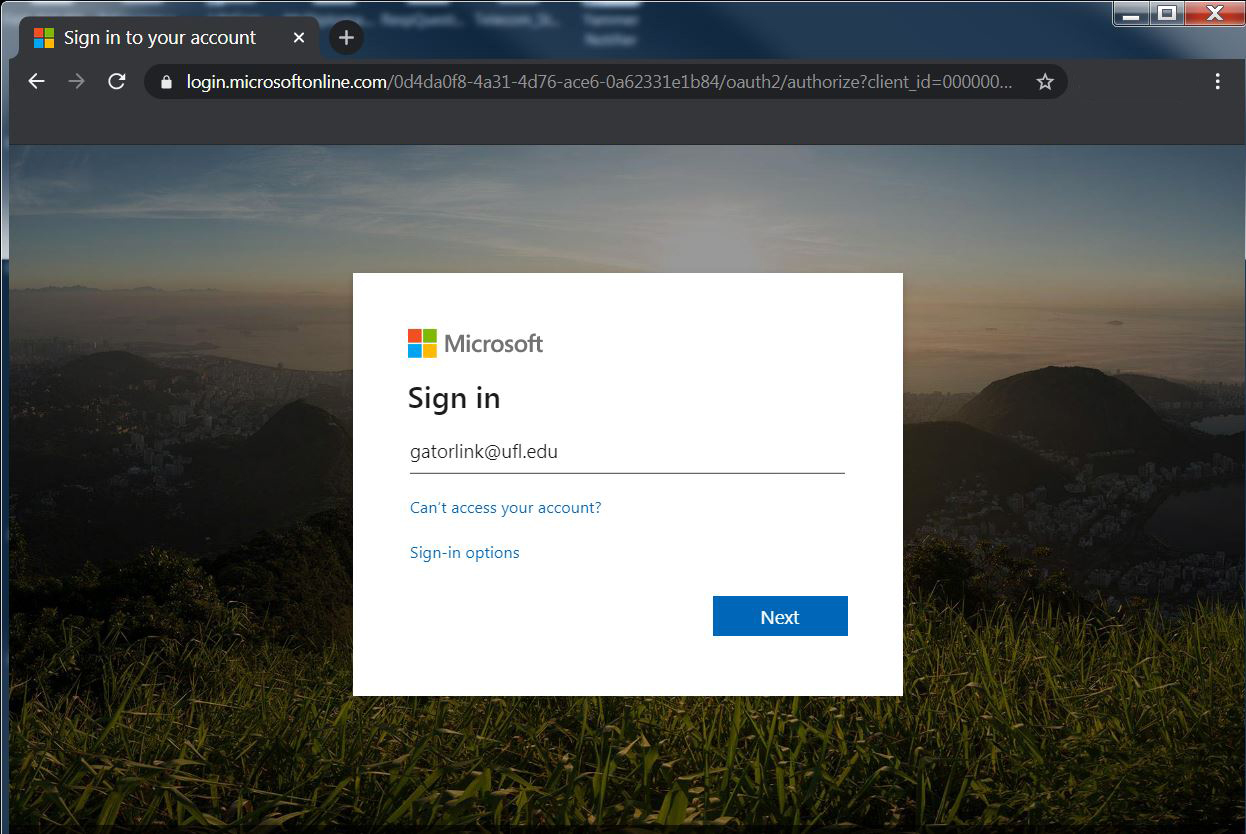
1. When prompted to enter Microsoft information as shown at right, enter your gatorlink user id information in the following format: gatorlink@ufl.edu.
Click next. |
 |
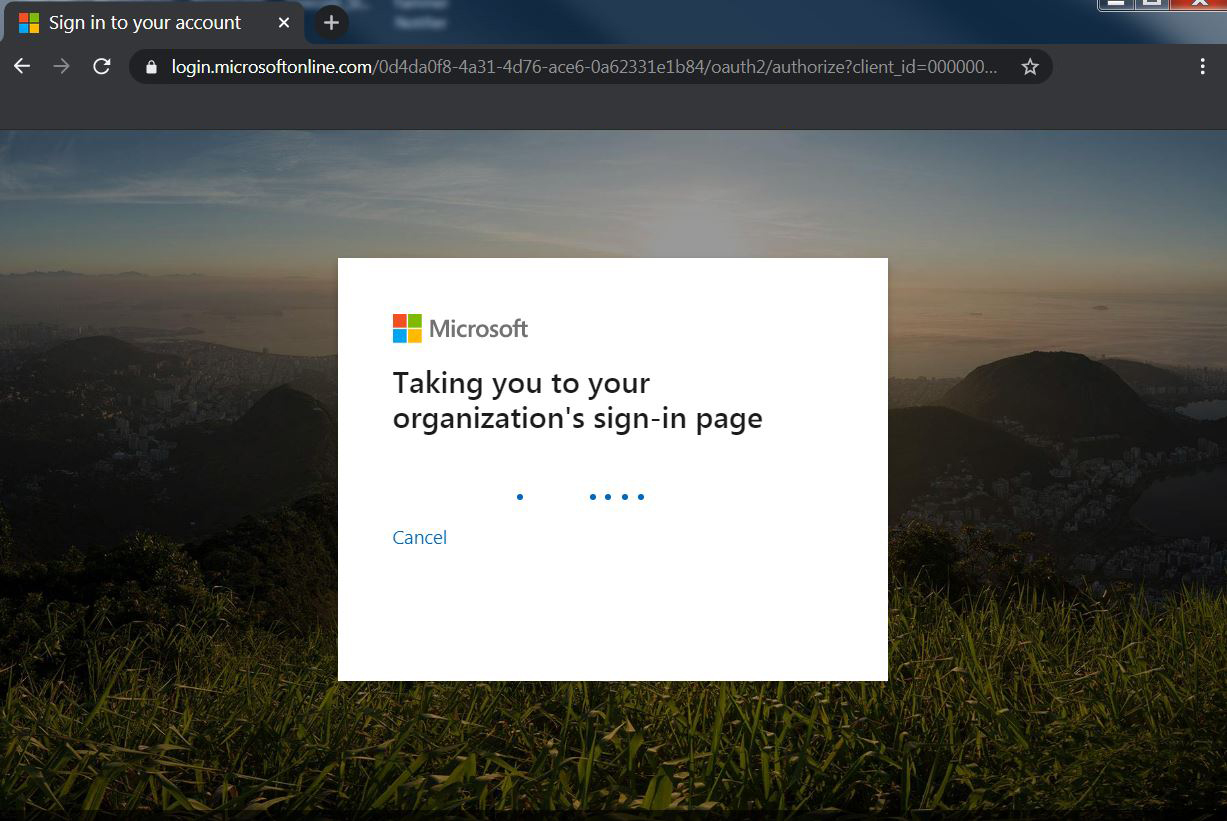
| 2. The system will take you to the organization (UF) sign-in page automatically. |
 |
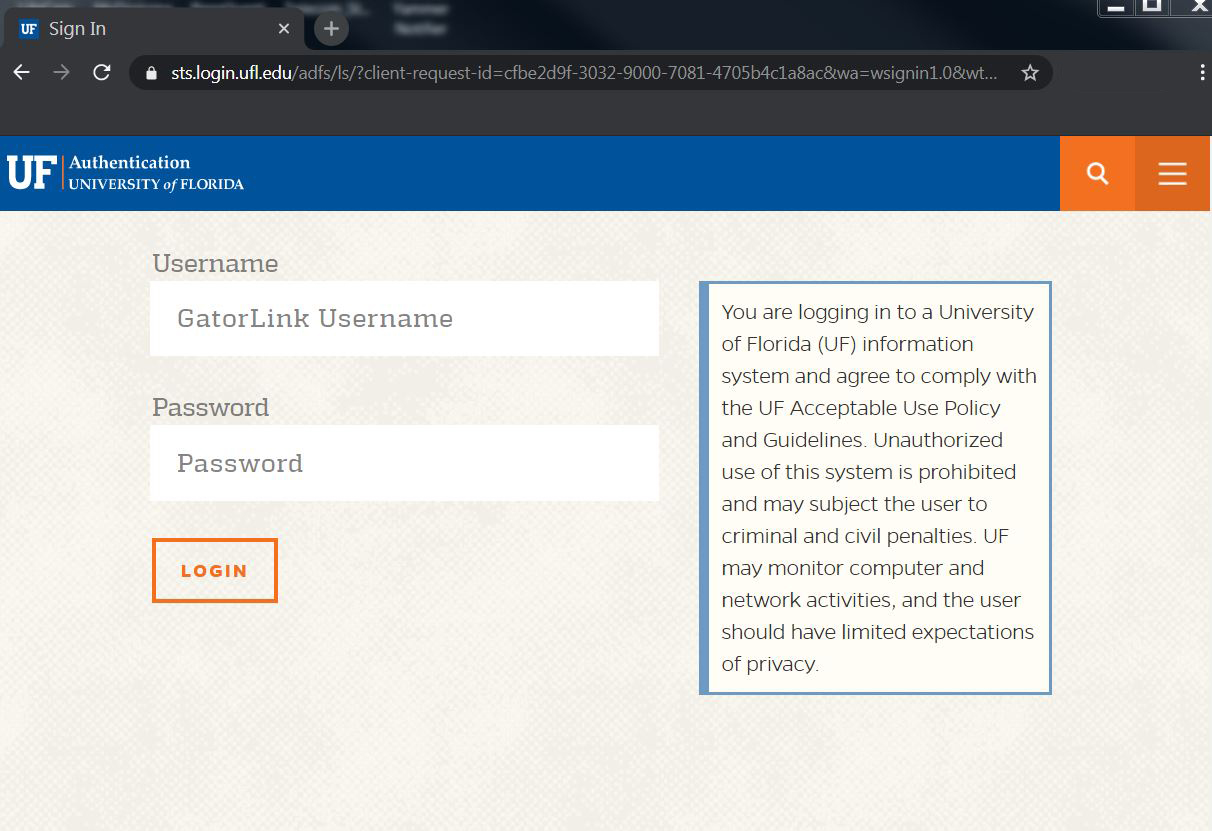
| 3. When prompted at the UF Authentication page, sign-in as you normally would. |
 |

