
Biohazard Project Registration
The purpose of the Biohazard Project Registration process is to identify and assess risks associated with work handling certain biological materials and to set control measures and practices to avoid exposure. Upon approval, the Biosafety Office and/or Institutional Biosafety Committee (IBC) will communicate specific guidance and requirements for the appropriate containment, procedures, and practices needed to prevent accidental exposures or unintended releases of these materials. Registrations are project specific.
The Biosafety Office no longer accepts paper registration forms (apart from Select Agent projects). All new project submissions, technical change amendments, and 5-year renewals are processed through the Gator TRACS Biohazard Project Registration module.
What Activities Requires Registration?
- Culturing or propagating an unknown/uncharacterized pathogen.
- Analysis of samples known or suspected to be contaminated with an infectious biological agent.
- The use of infectious biological agents pathogenic to otherwise healthy humans.
- Project will involve specimen collection (i.e. tissues, feces, saliva) from old world monkeys (i.e. macaques, baboons, etc.) which may be contaminated with herpes B virus.
- The use of cells immortalized with a virus (e.g. EBV, SV40).
- The use of toxins of biological origin that are either acute (LD50 < 100 µg/kg body weight) or export-controlled
- Experiments using recombinant nucleic acids
- Experiments using synthetic nucleic acids of any of the following: I) Designed to integrate in DNA; II) Replication competent; III) Encode a toxin with an LD50 < 100 ng/kg body weight.
- The use of recombinant or synthetic nucleic acid molecules, or DNA or RNA derived from such molecules, in human research subjects
Specific Inclusion Criteria include:
I. Recombinant nucleic acid molecules, or DNA or RNA derived from recombinant nucleic acid molecules, or
II. Synthetic nucleic acid molecules, or DNA or RNA derived from synthetic nucleic acid molecules, that meet any one of the following criteria:
a. Contain more than 100 nucleotides; or
b. Possess biological properties that enable integration into the genome (e.g., cis elements involved in integration); or
c. Have the potential to replicate in a cell; or
d. Can be translated or transcribed. - The requirement of federal or state permits (for possession, import, transport, or release)
How do I register my Project?
Login to Gator TRACS and follow the steps listed below. EH&S provides a step-by-step using guide on how to navigate the Biohazard Project Registration module, which can be downloaded by clicking below.


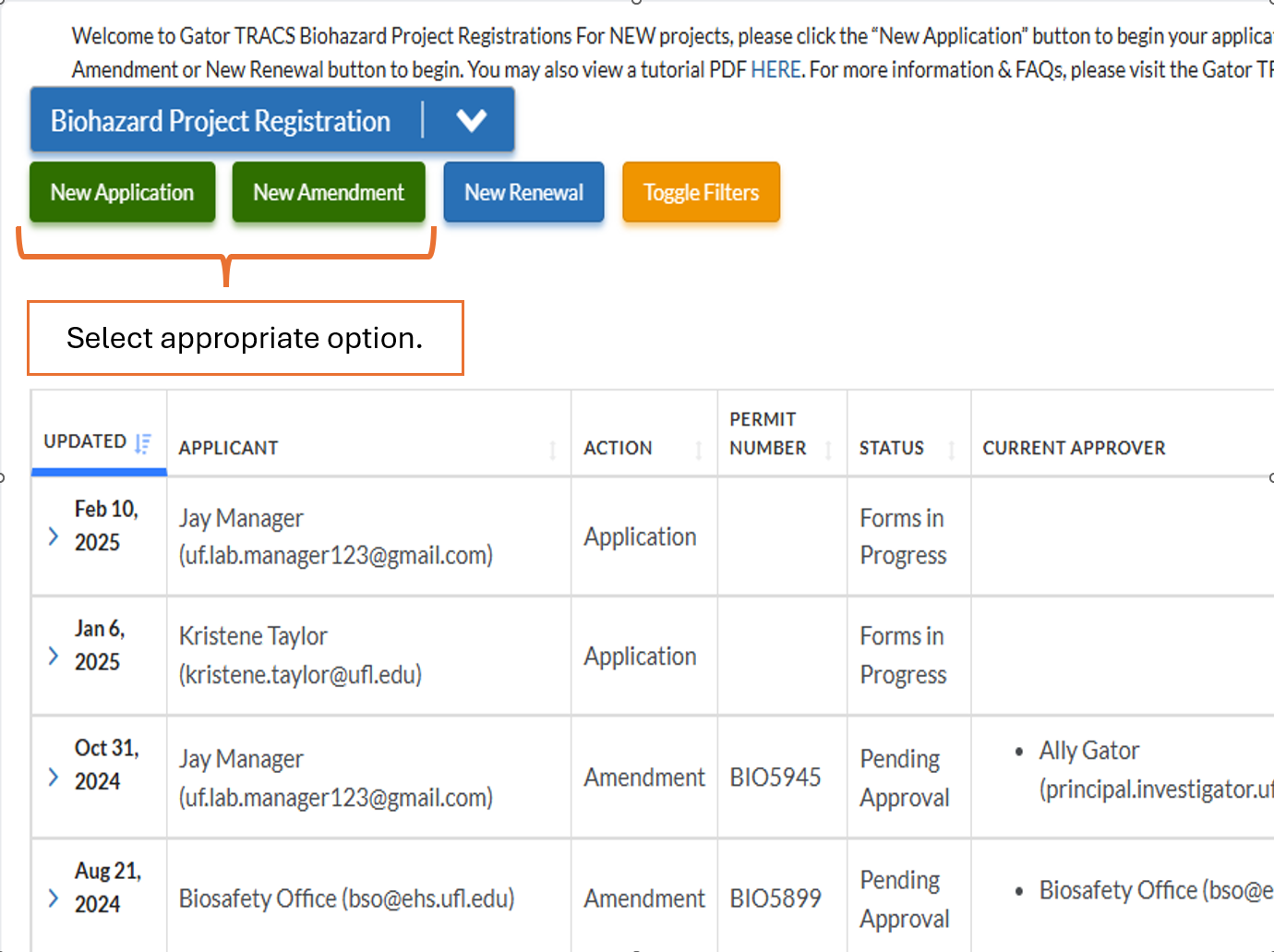

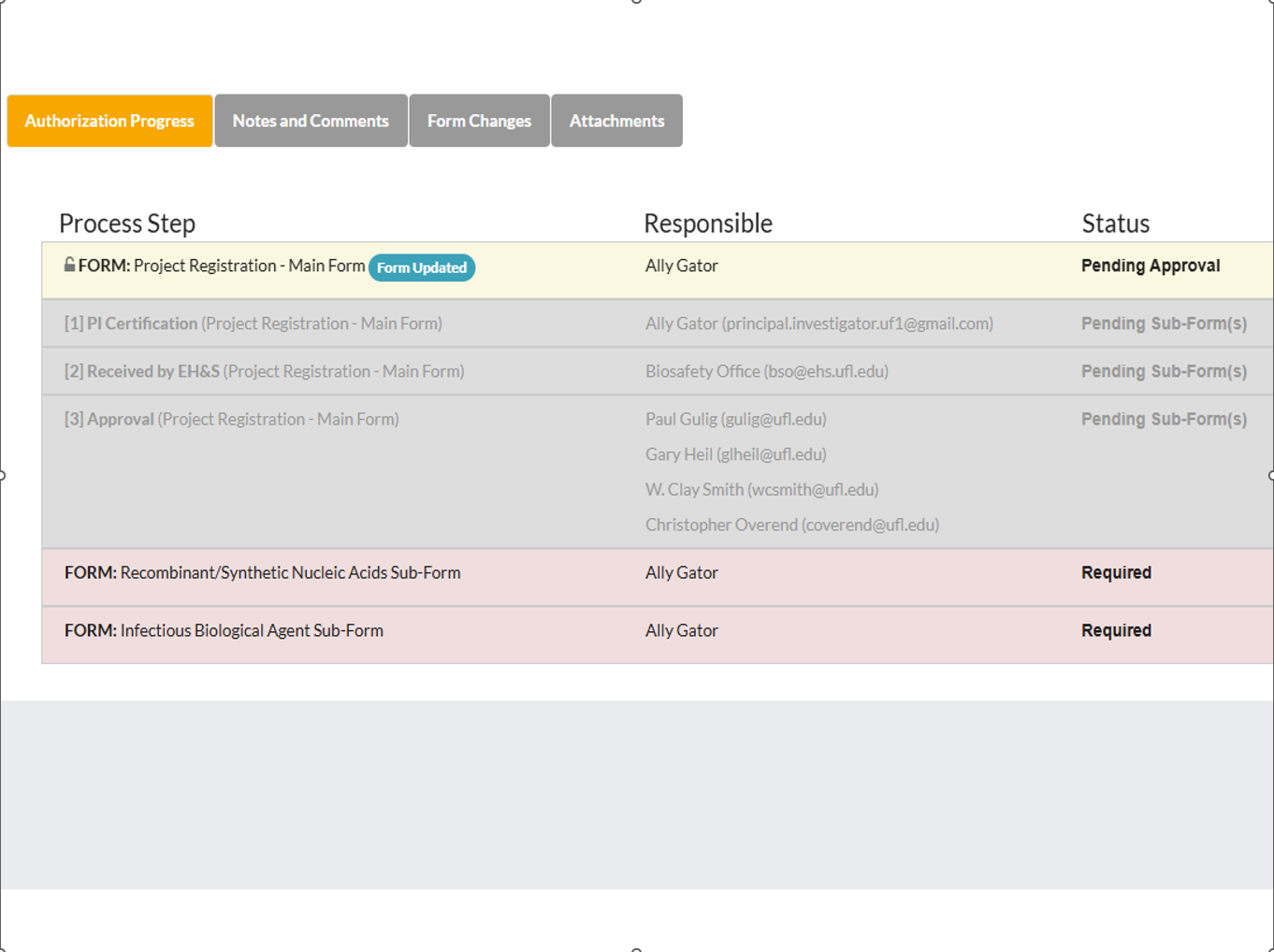
How to I Amend My Existing Project in Gator TRACS?
If your project is currently approved in Gator TRACS, you may amend the project by clicking “New Amendment” and selecting the appropriate project. Please review the User Guide and Quick Guide to Amendments for further instructions.
Additional FAQs
The transfer of projects from one investigator to another may only be done by the Biosafety Office. Please email BSO@ehs.ufl.edu to request this change.
Projects that include the use of rDNA/synthetic nucleic acid, projects that are non-exempt from NIH Guidelines, those that use CRISPR/Cas9 technology, are BSL-3 projects, or Select Agent projects require full Institutional Biosafety Committee (IBC) review and approval. The IBC meets twice a month (first and third Wednesday of each month).
What constitutes a project is at the discretion of the PI. In general, a project covers a specific scope of work with specific aims. An “umbrella” registration for all the work done in the laboratory does not provide sufficient detail for a useful risk assessment, whereas registration of individual experiments requires too frequent upkeep of the registrations. Consult the Biosafety Office for guidance as needed.
Project approvals generally take anywhere from 2 weeks to several weeks (6+ weeks). This is dependent on the complexity of the project, the quality of the submission, and the lab’s response time to EH&S/IBC comments and inquiries. These steps will help expedite your project approval:
In addition to system-generated notifications, the PI will receive at least two (2) email notifications from the reviewer in a 60-day period requesting a response to the comments in Gator TRACS. If a response is not received within the 60-day period, the Biosafety Officer will notify the PI that the application will be canceled.
Although the science will not be evaluated or critiqued, the Biosafety Office and Institutional Biosafety Committee require a thorough understanding of the scientific approach behind your project to assess the risks associated with handling certain biological materials. While describing safety mitigations is an essential part of the registration process, it is not enough to solely focus on safety measures without providing a clear understanding of the science behind the project. A biosafety coordinator will conduct a thorough review of the use of infectious biological agents, whether recombinant or synthetic nucleic acid molecules fall under NIH guidelines, and other potentially hazardous materials to determine the most appropriate biosafety level containment that facilitates safety for individuals working on the project. To do this, they require a detailed description of the science behind your project, including the goals, methods, and materials used. To minimize the number of questions you receive, it is recommended that you provide a clear and concise description of the scientific approach, including:
Visit the Select Agent webpage for more information and contact the Biosafety Office for further instructions.
