
Biohazardous Waste Disposal
Definition
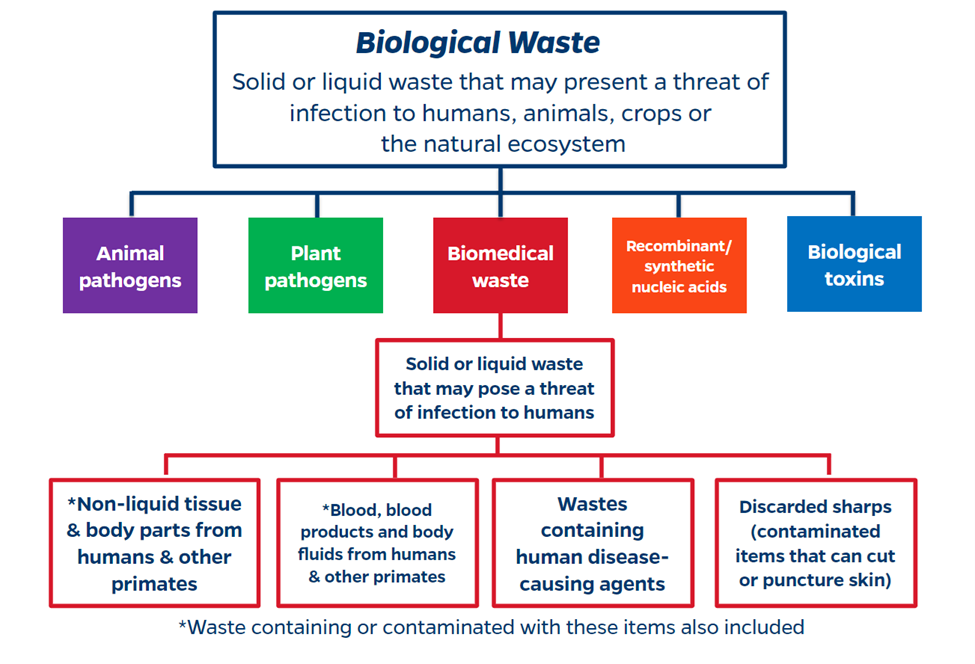
Biological waste is any solid or liquid waste that may present a threat of infection to humans, animals, crops or the natural ecosystem. Biomedical waste (BMW) is a specific subset of biological waste which refers to any solid or liquid waste, including discarded sharps that may pose a threat of infection to humans. Biomedical waste must be handled per the State of Florida Department of Health regulations and all employees who generate or package biomedical waste must take annual Biomedical Waste Training. The subsequent sections of this page explain how to handle each BMW stream (solid, liquid, sharp).
Non-Sharp Biomedical Waste
Solid Waste
Solid biomedical waste encompasses all non-sharp and non-liquid wastes such as contaminated paper towels, gloves, gauze, petri dishes, etc..
- Collect all solid biomedical waste in a leak-proof, covered container lined with a red autoclave bag. Cardboard biomedical waste boxes cannot be used for collection of non-inactivated waste.
- All BMW containers must have the international biohazard symbol and include the label “Biomedical Waste,” “Biohazardous Waste,” “Infectious Waste,” or “Infectious Substance.”
- Line the container with a red biomedical waste autoclave bag. Bags must meet the ASTM D 1922 and ASTM D 1709 standards for tear and impact resistance. Per State of Florida requirements, bags cannot be clear, orange, yellow or any color other than red. Labs are responsible for purchasing their own autoclavable bags.
- Label the bag with the Principal Investigator’s name, lab location (building and room), phone number and date that the bag was first put into use.
- Once ¾ full, loosely seal the bag with autoclave tape for inactivation. Best practice is to autoclave this waste at the end of each workday but at a minimum, it must be autoclaved at the end of each week.
- Transport the bags to the autoclave using an autoclave tray on a handcart. Place the tray in the autoclave prior to starting the cycle. Never place bags directly on the autoclave rack.
- Once autoclaved, follow the steps outlined in the Packaging Biomedical Waste for Pickup.
Liquid Waste
Liquid biomedical waste shall be inactivated by autoclaving or bleach treatment and then flushed down the drain with copious amounts of water.
Bleach Treatment:
- Add at least 1 part concentrated (8.25%) bleach to 13 parts liquid and mix well.
- Let the solution sit for a minimum of 30 minutes.
- Pour it down the drain while running water.
- Do not autoclave liquid waste that contains bleach as toxic chlorine gas may be generated
Autoclave Treatment:
- Collect the waste in a container that is autoclave-compatible (polypropylene or glass)
- Tightly close the container for transport to prevent spilling. Transport the container to the autoclave using a secondary container or autoclave tray on a hand cart.
- Prior to placing it in the autoclave, loosen the cap to allow for expansion.
- Autoclave using the appropriate liquid cycle:
- 121oC, ≥ 15 psi, 30-60 min
- Once cycle is complete and the liquid has cooled, dispose down the drain.
Sharp Waste
Any item that can puncture or lacerate the skin that is contaminated with biomedical waste must be disposed of using an appropriate sharps container. NOTE: all needles or needles attached to syringes (whether used or unused) must be disposed of as sharp BMW. This is independent of whether the needle has been contaminated with infectious materials.
- Biomedical waste sharps must be collected in a dedicated leak-proof, puncture resistant container with the appropriate markings.
- To prevent injury, never force sharps into a container, do not put fingers inside the container, do not remove needles from the syringe, do not bend or break a needle, and do not recap needles.
- Once ¾ full, close the box and label it with the PI’s name, location, and date.
- Alternatively, boxes must be dated when the first non-sharp item is placed in the container and closed and disposed of within 30 days. For this reason, non-sharp items such as wrappers and Kimwipes should not be placed in a biomedical waste sharps box.
- Sharp items used with infectious materials must be autoclaved prior to disposal. Once the closed container has been autoclaved, place the sharps box in the red bag lined biomedical waste box as outlined in the Packaging Biomedical Waste for Pickup
- Sharp boxes must be disposed of within 30 days of being closed.
- Sharp boxes can be requested from Building Services by contacting Work Management at 352-392-1121 or work-request@FacilitiesServices.ufl.edu. Once Work Management has been contacted, allow up to 2 business days to receive a call back from Building Services scheduling an appointment to pick up sharp boxes from the Health Science Center storeroom (AG129). Operating hours for appointments are Monday through Friday until 12:30 PM. There is a strict limit of 5 boxes per request.
Packaging and disposal
Inactivated waste must be placed inside a red bag lined biomedical waste fiberboard box. Boxes and liner bags are provided by UF’s biomedical waste vendor, Stericycle. The liner bags provided by Stericycle cannot be autoclaved.
Packaged biomedical waste boxes can be disposed using one of three methods:
Sealed boxes are placed in the hallway outside of the lab and are collected by Building Services personnel. This applies to the Health Science Center only.
Sealed boxes are transported by lab personnel to a dedicated collection room within their building to be picked up by Stericycle. Contact your building manager to inquire if your building has a Stericycle collection site.
Contact Work Management at 352-392-1121 or work-request@FacilitiesServices.ufl.edu to schedule a drop off appointment at the Health Science Center Stericycle trailer. Allow up to 2 business days to receive a call back from Building Services scheduling an appointment. Once the appointment is scheduled, lab staff must transport sealed boxes to the Health Science Center Stericycle trailer for disposal.
Supplies
Labs are responsible for purchasing their own autoclave bags. Stericycle cardboard boxes and liner bags are provided free of charge by either Stericycle or Building Services through one of the following methods:
Building Services will deliver boxes and liner bags to labs within the Health Science center when picking up packaged waste. You may also call Work Management at 352-392-1121 to request boxes.
Stericycle will deliver boxes and liner bags to buildings with a dedicated Stericycle pick-up stop. Contact your building manager to inquire if your building has a Stericycle collection site.
All others must schedule a pickup from the Health Science Center storeroom (AG129) by contacting Work Management at 352-392-1121 or work-request@FacilitiesServices.ufl.edu. Please allow up to 2 business days to receive a call back from Building Services scheduling an appointment. Operating hours for appointments on Monday – Friday until 12:30 PM.
Transport
The transport of biomedical waste across campus must meet specific requirements:
- Do not transport more than 25 lbs. at one time.
- Transport boxes using a hand cart or state vehicle. Personal vehicles are not allowed.
Non-Biomedical, Biological Waste
All labs are encouraged to minimize biomedical waste generation whenever possible. Many times, biological waste not classified as biomedical waste ends up in the biomedical waste stream. This results in higher disposal costs for the institution and greater impacts to the environment as biomedical waste treatment is an energy intensive process. Solid biological waste that does not pose a threat of infection to humans can be discarded in the regular trash or clean labware box after steam or bleach inactivation. It is not recommended that this waste be disposed of as biomedical waste unless inactivation of the waste is not possible or if segregation of the waste from true biomedical waste is difficult. Non-biomedical, biological waste includes non-infectious recombinant/synthetic DNA, microorganisms not infectious to humans (risk group 1 agents, including plant and animal pathogens), and presumed healthy animal products (excluding non-human primate). Similar to biomedical waste, liquid waste must be inactivated using an autoclave or bleach and poured down the drain following inactivation.
