Hazardous waste accumulation must follow the University of Florida guidance presented here. UF guidance is based on Federal and State regulation and must be followed closely in order to avoid harm to human health or to the environment. Hazardous waste accumulation throughout campus is subject to unannounced Federal and State inspection. Non-compliance can result in both civil and criminal penalty.
Satellite Accumulation
Hazardous Waste at the University of Florida must be initially accumulated at the point of generation, in designated “Satellite Accumulation Areas,” or SAAs. The EPA has simplified hazardous waste management at this level and the requirements are summarized in the Satellite Accumulation Area Requirements and Illustrated in the poster below.
Satellite Accumulation Area Requirements
The following rules apply to all UF Hazardous Waste storage areas, called “Satellite Accumulation Areas,” or “SAAs.” A
printed copy of these rules is required to be posted in the immediate vicinity of each SAA to designate that the area is set aside for Hazardous Waste storage.
- MARK ALL CONTAINERS conspicuously with the words “HAZARDOUS WASTE.” AND INDICATE the HAZARD(S) present (i.e. Flammable, Oxidizer, Corrosive, Reactive, Toxic)
- LABEL ALL CONTAINERS ACCURATELY, indicating the constituents and approximate percentage of each. The concentration of the constituents must add up to 100%.
- LIMIT the satellite area waste volume TO no more than 55 GALLONS of waste, OR 1 Kg solid (1Qt liquid) of a “P-LISTED” waste at any one time.
- CLOSE all containers during accumulation except when necessary to add wastes. Do not overfill containers. Leave adequate headspace for expansion.
- SEGREGATE containers of incompatible waste to prevent reactions. Ensure waste is compatible with other wastes in the container, and with the type of container it is stored in.
- NO BIOHAZARD waste and NO RADIOACTIVE waste may be mixed with or stored in the same location(s) as Hazardous Waste.
- Satellite Accumulation must be AT OR NEAR the POINT OF GENERATION; waste must be UNDER THE CONTROL and supervision OF THE GENERATOR.
- TRAIN ANNUALLY all personnel who handle or generate hazardous waste. The Lab Waste Manager must complete EHS809 ILT. Other staff may complete EHS809 OLT.
- PREPAREDNESS: ALL SAAs must have access to a: FIRE ALARM, FIRE EXTINGUISHER, SPILL KIT, COMMUNICATION DEVICE (Telephone or Two-Way Radio), and staff must maintain adequate AISLE SPACE.
Downloadable SAA Poster
Downloadable Monthly Self Audit Sheet
Labeling
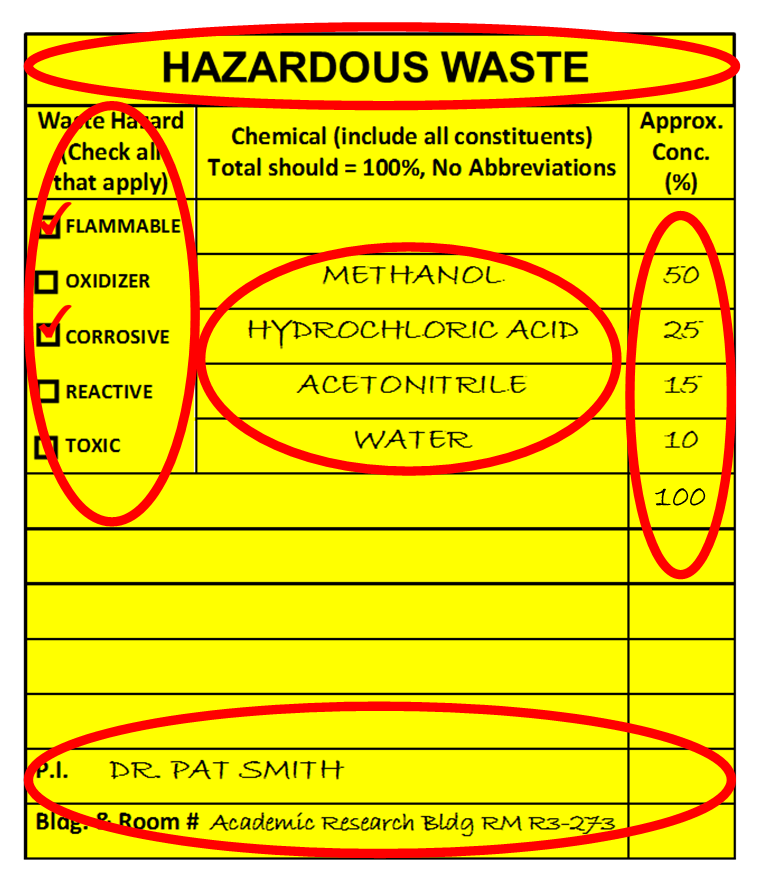
All Hazardous waste must be labeled and include the following:
- “Hazardous Waste”
- Indication of Hazard (check all hazards present-must match content)
- Chemical Constituents (% must add up to 100 prior to collection)
- Generator and Location Information (P.I./Bldg/Room #)
- Unwanted/Unusable original product must be labeled if Hazardous Waste
- Non-Hazardous original products Do Not require additional labeling
- Request Hazardous Waste using the link or by calling 352-392-8400
You may generate custom labels using the templates below:
Containers

- Chemically compatible with waste
- DO NOT use metal containers for corrosive liquids
- Use ONLY polyethylene containers for Hydrofluoric Acid
- Immediately address damaged or leaking waste containers.
Container requests can be made by using the chemical waste pick-up request form
Segregation

Hazardous wastes must be segregated by hazard to prevent unintentional mixing and dangerous reactions. The hazard indication requirement on the updated label can assist in the segregation but also highlights these incompatibilities. Make sure your labeling matches your segregation.
Disposal
Absolutely no chemical hazardous waste may be discharged to sanitary drains or disposed of into the environment (via “normal” trash cans, dumpsters, etc.).
 All chemical hazardous waste must be disposed of through EH&S. Lab waste managers may request waste collection by submitting a Chemical Waste Pick-Up Request Form online.
All chemical hazardous waste must be disposed of through EH&S. Lab waste managers may request waste collection by submitting a Chemical Waste Pick-Up Request Form online.
Prior to submitting this request, the waste manager must ensure that all containers to be collected are tightly capped and properly labeled.





